(9 PM – promoted by TheMomCat)
We hear a lot about trans fats in food and the negative health effects of them. However, most folks without a background in chemistry do not really know what that means. Tonight the object is to clear that up, and to point out sources that are high in them so they can be avoided.
Contrary to the opening statement, not all trans fats have deleterious health effects. There are a couple that seem to be beneficial, but unfortunately they are sort of rare. They are also some of the few trans fats that occur naturally. By a huge margin, most trans fats consumed are artificially produced, and we shall get into that as well.
To understand the topic well, a chemistry lesson will first have to be given. However, this IS Pique the Geek!
We can either start out with explaining what fats are or what trans means. Let us start with trans first and then go on to fats. In chemistry, the element carbon can bond not only with other kinds of atoms, but also with other carbon atoms. These bonds can be either single bonds, double bonds, or triple bonds. In large molecules it is possible to have more than one of each. Triple bonds are comparatively rare in natural products, so we shall limit our discussion to single and double ones.
A carbon-carbon single bond is a sigma bond, where electron density is built up directly betwixt two carbon atoms. Here is a representation of a sigma bond, with electron density indicated by the red color, the deeper the color the higher the electron density:

An extremely important characteristic of sigma bonds is that there is free rotation of the carbons atoms around the bond. This will make more sense in a few minutes.
A carbon-carbon double bond consists of a sigma bond plus a pi bond. A pi bond consists of electron density being built up over and under an imaginary plane that bisects the sigma bond in the line betwixt the two carbon atoms. This bond is thus in two halves, above and below the sigma bond with no significant electron density from the pi bond directly betwixt the two carbon atoms. Here is a representation of a pi bond being formed from two p atomic orbitals:

An extremely important of pi bonds is that there is not free rotation of the two carbon atoms when a pi bond is present, because as the bond is twisted the bond has to weaken and then break, and that takes a lot of energy or a catalyst.
Now let us consider the simplest hydrocarbon that can by trans. First, however, let us look at the all single bond cousin, butane, C4H10.
Here is the full structural formula:
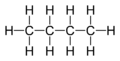
Here is the representation that organic chemists prefer, the skeletal formula, where each “end” of the zigzag line represents a -CH3 group and each bend represents a -CH2- group:
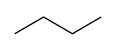
You can understand why we prefer to use this representation, because all of the information is conveyed, and a rough approximation of the actual geometry of the molecule is presented.
Here is a three dimensional look at the most stable configuration of butane:
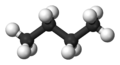
Note that the hydrogens, represented by the white balls, are as far apart as possible, thus the reason for this being the most stable configuration. However, because of free rotation, they can twist closer together and at room temperature all of the carbon-carbon bonds are rotating. This most stable configuration becomes “locked” only at very low temperatures and can be seen to do so by NMR.
Now let us look at butene, which has one carbon-carbon double bond. There are actually three straight chain C4, but only two of them are of interest for reasons that quickly become obvious when you see these two structures. First comes trans-2-butene:
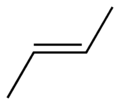
Here is the 3D view:
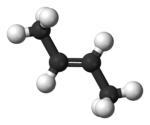
Note that the bulky end methyl groups are as far apart from each other as possible. Trans is Latin for, roughly, “across from”.
But there is another form of 2-butene. Remember, carbon-carbon double bonds do NOT allow free rotation. This is called cis-2-butene, and here are the representations of it:
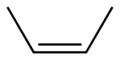
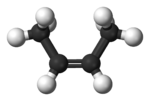
Note now that the two bulky methyl groups are much closer to each other then in the trans form. Cis is the Latin for roughly “on the same side”. Remember, there is no free rotation around the double bond, so these are two completely different chemicals with differing boiling and melting points, and more importantly, different enthalpies of formation. In general, trans molecules are a bit more stable than cis ones, and this is important later.
You may have heard the terms Z and E in place of cis and trans, Z being the abbreviation for the German zusammen, meaning “together” and E being the abbreviation for entgegen, meaning “apart”. Newer usage is favoring Z and E, but cis and trans are still very widely used.
Now that we have had our lesson in stereochemistry, it is time to discuss fats. A fat is generally a fairly large molecule, actually a condensation product betwixt one glycerol (aka glycerine) molecule and three fatty acid molecules. Now, there is only one glycerol:
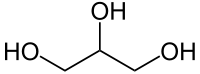
As you can see, it has three carbons, each with one or two hydrogens on them, and each containing a hydroxy group, -OH. This makes glycerol an alcohol, because in organic chemistry alcohols are hydrocarbons containing at least one hydroxy group and no other kinds of special groups. The hydroxy groups are reactive, much more so than carbon-carbon for carbon-hydrogen bonds. This is important.
There are literally hundreds of fatty acids. However, all fatty acids have two features in common: they all are fairly long (at least four carbons, usually quite a few more), and all contain the carboxyl group, -COOH. These groups are even more reactive than hydroxy groups. It is this group that makes vinegar taste sour and gives it its unique odor. Another name for these acids is carboxylic acids, and that is more general than fatty acid, because fatty acids are the subset of long chain carboxylic acids.
Now, alcohols and carboxylic acids react readily with each other to form organic compounds called esters. Everyone is familiar with them. Here is an experiment that you can do at home: take a few drops of vinegar (and acetic acid solution in water) and add around a teaspoon of vodka (an ethyl alcohol solution in water) and mix them. You may need to heat it just a bit. Soon you will smell an odor different than either vinegar or vodka. You just make an ester, ethyl acetate! Here is the chemistry:
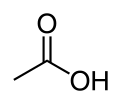 +
+ 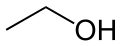 yields
yields
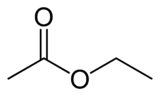 +
+ 
In words, it is one molecule of acetic acid plus one molecule of ethanol yields one molecule of ethyl acetate plus one molecule of water. This is an example of a condensation reaction, where two molecules come together to make a bigger one with the elimination of a small molecule. The red lettering on water are the HOH bond angle and the OH bond length.
Fats are made when three molecules of a fatty acid are condensed with one molecule of glycerol to form a triglyceride (fat) and three molecules of water. This is an extremely common reaction. Interestingly, we do the exact reverse when soap is made from fats, with lye (sodium hydroxide) breaking the bonds betwixt the glycerol and each fatty acid and forming the sodium salt of the fatty acids, soap.
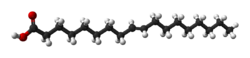
Now let us look at a few typical fatty acids. One very common one is stearic acid, obtained from beef tallow. Here are the structural formulae for it:
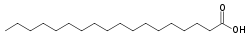
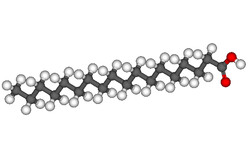
Note that stearic acid has no carbon-carbon double bonds. These kinds of fatty acids are called saturated, because with no carbon-carbon double bonds mean that no hydrogen can be added. When fats are formed with lots of these saturated fatty acids, they are call, obviously, saturated fats. Saturated fats tend to increase LDL, the “bad” cholesterol and decrease the HDL, or “good” cholesterol in the blood, which is bad for the circulatory system.
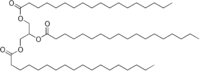
A fat, or triglyceride, composed of only steric acid and glycerol has this structure:
Now let us compare this to the monounsaturated fatty acid oleic acid, which is very good for the circulatory system (it is the primary fatty acid in olive oil):
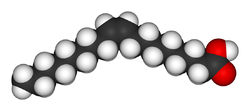
Both of these fatty acids have 18 carbons, but oleic acid has two fewer hydrogens than has stearic acid because of the double bond. This is called monounsaturated because it has only one carbon-carbon double bond. But look at the difference in the geometry. Steric acid is a straight, more or less, chain, but oleic acid has the carbon-carbon double bond near the middle. This is a cis double bond, so you can see that the two bigger parts of the molecule are on the same side of the double bond.
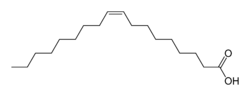
Now let us look at the trans form, called elaidic acid:
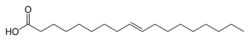
Note how much more like the unhealthy stearic acid it looks than does oleic acid. It turns out that elaidic acid is even worse than stearic acid towards the circulatory system. The reasons are unclear, but one leading candidate is the idea that we have enzyme systems to deal with cis fatty acids that do not function well on trans ones, because almost all natural fatty acids are cis, and we have not had millions of years to become accustomed to trans acids.
It turns out that elaidic acid does occur in nature, with around 0.1% of all of the fatty acids in milkfat being represented by it. That is not really enough to be a problem. The problem with milkfat is that it is highly (around 62%) saturated.
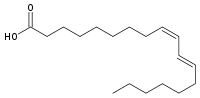
So where do we get trans fats? Until early in the 20th century, from ruminant animals, where a few types are produced in their rumen. Interestingly, these natural ones seem to have positive health properties. One is conjugated linoleic acid which has two carbon-carbon double bonds one cis and the other trans. Here is the formula:
Another is vaccenic acid, which has also shown to have positive effects in animal models:
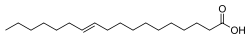
Note that is looks just like elaigic acid except the carbon-carbon double bond is one carbon further away from the carboxyl group. What a difference one position can make in biochemistry!
Except for these two trans acids, and possible a very few more, the general consensus is that trans fatty acids are bad for people. Since only the “good” ones occur naturally, why the big deal about them? “Modern” technology provides us with the source. It turns out that unsaturated fatty acids, particularly cis ones, are reactive with oxygen and tend to go off faster than saturated fatty acids and trans unsaturated ones.
It also turns out that cis unsaturated fats tend to be liquids at room temperature and that saturated fats and trans fats tend to be solids. This might not sound like a big deal, but for baking it is critical. Liquid fats make very poor pastry crusts, causing them to be sandy rather than flaky. If anyone is interested in why, ask in the comments. It is after 8 PM Eastern and I need to get this in the can fast.
Additionally, animal fats, the source of highly saturated fats that are stable and good for baking, are much more expensive than fats from vegetable sources in many cases. What was needed was a way to make liquid vegetable oils behave more like solid animal fats. Over 100 years ago a German chemist discovered a way to add hydrogen to unsaturated fats and thus stabilize and solidify them. This process is called catalytic hydrogenation and it makes shortening from oils.
Remember that I defined “unsaturated” to mean deficient in hydrogen. In hydrogenation, a liquid oil, like cottonseed oil, is put in a pressure vessel and hydrogen introduced into the vessel. Nothing happens, unless you also add some very finely powdered nickel to the mix. The unusual electronic configuration of nickel interacts with the carbon-carbon double bond(s) and weakens them such that hydrogen can be added, forming a saturated product. Well, hydrogen is not exactly free, so just enough is added to solidify the oil to the degree desired. As a matter of fact, professional bakers can obtain shortenings custom hydrogenated to produce a melting range for specific products.
Well, the nickel does weaken the carbon-carbon double bond, to the point that free rotation is possible (remember that I said earlier that the lack of free rotation was important). If not enough hydrogen is added to the oil to make it fully saturated, the less thermodynamically stable cis form reverts to the more stable trans form and gets “frozen” as trans when the reactor is emptied. Also recall that I previously said that it was important to remember that trans is in general more stable than cis. Thus, in addition to converting healthful cis fatty acids to less healthful fully saturated fats, what remains unsaturated is now almost all trans! Since trans fats behave physically more like fully saturated fats, shortenings are nice and hard, and are superb for baking.
Shelf life of commercially produced baked items is also increased due to the lower reactivity with oxygen, and the products have better “mouth feel” than those made with cis unsaturated fats. The cost is also lower, since animal fats are replaced by plant derived ones.
There is a trend to reduce the levels of trans fats in commercially prepared foods, and this is done in several ways. One is to use fully hydrogenated oils, where all of the carbon-carbon double bonds are saturated and thus cis and trans have no meaning. These fully hydrogenated oils are then blended with nonhydrogenated oils to produce a product that has similar baking properties to the older products. However, shelf life is shorter because of the presence of cis fats in them. Remember, cis fats are much more oxygen reactive than either fully saturated fats or trans unsaturated fats.
So how much trans fat is in food? At the extreme, some partially hydrogenated oils can contain up to 45% on total fat basis as trans fat. That is 10 times the amount in milkfat! Typically, old style shortenings were about 30% trans fat on a total fat basis, and margarine that has not been reformulated to the new trends is around 15% on a total fat basis. Things have improved, but unnatural trans fats are still with us and will be for the foreseeable future.
So, how to avoid unnatural trans fat? Read labels. Also, so not be fooled with the statement, often prominently displayed, that says “Zero grams trans fat per serving.” The Food and Drug Administration, and I think because of pressure from Congress, had defined “Zero grams” as less that 0.5 gram trans fat per serving. How many servings does it take to get a lot? Well, for a 2000 calorie diet, 20 grams of saturated fat is the recommend maximum. Assuming that trans fat is equally bad (and most studies indicate that trans fat is much worse) as saturated fat, ten servings of foodstuffs at the maximum 0.5 gram per serving to be qualified to be “zero” would provide five grams of trans fat! The definition of “serving” is also up to the manufacturer.
I am looking at the label for peanut butter bars from a big box store (their own store brand). It says “zero” for the amount of trans fat “per serving”. Now, their definition of serving is “one bar”, but they package them cellophane wrappers containing two bars! Thus, it is possible to get almost one gram of trans fat by eating two bars. There are other tricks.
One trick that I recently noticed was using the term “hydrogenated vegetable oil” rather than “partially hydrogenated vegetable oil”. This is sort of devious. “Partially hydrogenated vegetable oil” is a sure sign of trans fat, but “Fully hydrogenated vegetable oil” by definition has none at all. By using the term with no qualification makes it hard to say. Here are my recommendations:
Look at the ingredient statement. If you see “partially hydrogenated vegetable oil”, put it back. If you see only “fully hydrogenated vegetable oil”, there should be no unnatural trans fats. If you see “hydrogenated vegetable oil”, be very dubious of the trans fat content. Personally, I try to eliminate as much trans fat as possible because I am already at risk for circulatory problems because I smoke tobacco.
How bad are trans fats for you? The consensus is that the unnatural ones are pretty bad, but it has a lot to do with many variables. My grandmother was a fan of the most famous name in shortening, and she lived to be 100 and a half. My mum was also a fan, and died of cardiac problems at 76. My maternal grandfather died at 53 of heart problems, and he ate what aforesaid grandmother cooked. So go figure. The point of this piece is not to debate the health effects of trans fats, but rather to explain what they are and how we get them. I hope that I accomplished that goal.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this greasy piece! And even though Herman Cain realizes that he has no chance at the nomination, let alone winning the general elections when he reads me say it, I always learn much more than I could possibly hope to teach writing this series. So please keep those comments, questions, corrections, and other feedback coming via the Comment Section, the best parts of my writings. I shall hang around as long as comments warrant tonight, and shall also return tomorrow for Review Time after Keith’s show. By the way, he still needs to call me about that Science adviser gig. Bill Nye, indeed! Nye also goes of The Fox “News” Channel, by the way.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Daily Kos, and

3 comments
Author
a filling subject?
Warmest regards,
Doc
Dad was born in 1922, Ma in 1926 and as such I have argued that she and Dad spent more years eating more natural foods because they spent more time before the scientific revolution which may be loosely attributed to he 1950s. They ate less processed foods, had less exposure to that vast list of relatively new MSDS chemicals in addition to exposure to again relatively new components added to the electro-magnetic spectrum.