(9 am. – promoted by ek hornbeck)
Last week we discussed the Deepwater Horizon blowout and the resultant huge oil spill, and will continue the discussion tonight. By the way, the title of this piece represents the official NOAA estimate of the release. Some estimates are as much as five to ten times this amount, but the NOAA estimate one is the official one, so we will use it.
It really makes little difference, because even the official estimate is huge. Since this happened on 20100420, with the rig sinking and presumably destroying the riser on 20100422, at the official rate for the 18 days now 3.7 million gallons have been released.
Before we get started, please allow me the opportunity to wish all of the mothers reading this a very HAPPY MOTHERS’ DAY! I had a wonderful one, and my boys still have a wonderful one.
This is around one-third the size of the Exxon Valdez spill in Alaska at present. Only about 10% of the spilt oil was recovered, and the remaining 90% is estimated to be disappearing at about 4% per year, leaving lots of oil in the sound. Now, Rush Limbaugh says that Prince William Sound is pristine again. Since he is an internationally recognized petroleum engineer, I guess that the agencies and environmentalists still monitoring Prince William Sound are just misguided.
I maintain that the damage in the Gulf of Mexico likely will by much worse environmentally because of the difference in temperature of the surface water and the land. In Alaska, with a much lower average temperature, the oil tends to stay in place. In addition, Alaskan oil tends to be heavy and gulf oil much lighter, so gulf oil will tend to float rather than sink. This means that gulf crude will also evaporate faster than Alaskan crude, especially in the temperatures in the gulf region.
This evaporation is why “tar” balls are now washing up onto the shore. The volatiles evaporate fast, leaving the heavier material that looks like tar. This reduces the volume of the oil that will reach the shore, but the chemical components in the heavier residues are actually more toxic in many cases than the volatile materials. The worst case is for oil to wash into the wetlands and coastal areas and then the volatiles evaporate, leaving a toxic, hard to remove residue. This is likely what will happen, and the longer it takes to cut the escape of oil the worse that will be.
We also talked last week about the funnel. BP actually fabricated and placed the funnel faster than I had expected and it failed. Now, I am not a petroleum engineer, but simple physics will tell you what went wrong. This seemed so obvious to me that I did not even mention it last week, and in retrospect should have in case someone actually working the situation would read it. In short, they left the valve open (if they even installed a valve) between the “ceiling” of the funnel and the pipe to direct the oil to the collection barge.
Why is this important? One has to look at the conditions at 5000 feet in the gulf. The temperature at that level is around 2 degrees C, just above the freezing point of water. Not a problem for water freezing in the pipe. However, it is not water ice that clogged up the pipe. It actually is methane hydrate (also called methane clathrate). This is a cage compound of methane and water where methane occupies the center of a cage consisting of water molecules.
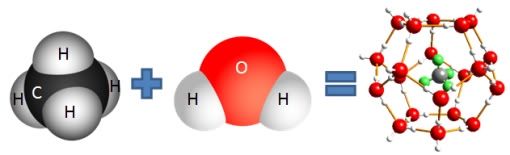
Methane hydrate
Actually, there are usually three or four methane molecules in the cage, for a molar ratio or around 5 or 6 waters to 1 methane.
At atmospheric pressure, methane hydrate freezes at about the same temperature of water. However, this is not the case under pressure. At 5000 feet the pressure due to the water is about 150 atmospheres (at the surface, obviously the pressure is one atmosphere). Under these conditions methane hydrate freezes at 18 degrees C! OF COURSE the pipe froze. Now, this methane hydrate was not there originally. It forms as the methane dissolved in the oil reacts with the water, but the hot oil will not allow it to form close to the leak, but the methane has to get far enough away from the oil to cool down sufficiently in the cold water. What they did was drop the funnel, full of 2 degree water, down onto the leak and it immediately started forming hydrate in top of the funnel and in the pipe.

Funnel being lowered
The relatively small diameter of the pipe is efficiently cooled by the cold seawater, so it can never thaw, even by the warm oil. However, if the funnel had been dropped with the valve closed and left in place until the oil warmed it up, and then the valve opened just enough to allow MOST of the oil but not quite all of it, little or no hydrate would be formed. The reason to allow a little to leak from the bottom is to keep seawater from infiltrating into the funnel. The funnel would be warm enough, but the pipe (and the long pipe leading to the surface) will still be efficiently cooled by the seawater, so the pipe would freeze up again if a significant amount of water were to be in it.
BP says, well, we built too big a funnel. That is nonsense. They built a funnel of ill design, and until they devise a way to throttle the flow so that hydrate does not form in the pipe, it does not matter what size funnel they build. The trick is to allow the warm oil to fill the funnel, allow time enough for any hydrates to thaw, then attach the long pipe leading to the surface, and pump out that pipe. Then open the valve to allow essentially pure oil up the initially DRY pipe. It is not really that complicated, at least in theory.
Whilst we are talking about the funnel, almost every TeeVee newsreader says that this funnel will be used to siphon the oil into a collection barge. Siphoning involves moving a liquid from a higher elevation to a lower elevation using air pressure. Your commode siphons water from the bowl to the drain leading to the sewer. Siphoning NEVER involves raising a liquid to an ultimate higher level, although it may pass through an intermediate higher elevation.
BP has several other contingency plans. One is to inject methanol into the funnel to dissolve the hydrate. Methanol is pretty toxic, and is water soluble, so this might not be the best approach. Some of that fuel grade ethanol would be a less toxic alternative. By the way, one of the citations in Alaska that BP received was for a methanol spill, so they are very familiar with hydrate formation. They have to wash out the pipelines in the cold climate of they clog with hydrate and clog, so this is not a new concept for them.
Another idea is to tap into the riser itself and attach a new connection to a pipe to direct the flow to the collection barge. That is conceptually a good idea, since the oil, like with the funnel, not only would not pollute, but would also be conserved.
Another concept is to shoot debris into the blowout preventer to clog it and staunch the flow. That would be followed by injections of concrete to cap the preventer, hopefully for good.
All of these approaches will buy time to drill relief wells to reduce the pressure on the damaged structures and allow a more permanent solution. It is always a crapshoot as to when damaged structures like that will let go again. Of course, a permanent cap on the existing well will have to be put in place, but if the leaks are mitigated first, the proper time and planning can be used to do it right. Interestingly, little mention is being made of relief wells these days.
The answer to the question as to what actually happened to cause the blowout is not yet clear. Obviously, a sudden, severe pressure rise occurred and the blowout preventer failed. By the way, blowout preventers are old technology, going back to the 1920’s for some designs. I will be extremely interested to learn why it failed to function. It is not that is was not robust enough to contain the pressure. It failed to operate. The way this particular rig was designed, instruments in the control room constantly monitor working pressures. Operators man the screens and can trip the blowout preventer manually if an event occurs. In addition, it also is supposed to activate if the telemetry from the control room fails. As a Kossack said last week, the acoustic trigger (that was not present) would not have helped, because all it does is activate the blowout preventer. Even the deepwater manual triggers did not activate it when the submersibles were dispatched. The blowout preventer failure is the immediate cause of this disaster, and a complete investigation as to why it did not function certainly will be made.
In an ironic note, several bigwigs from BP were on the platform when the blowout occurred to celebrate the wonderful safety record of the project. Just for some perspective, at this time last year there were 39 fires or explosions involving gulf oil rigs, so this is not as rare as the oil industry would have us believe. This one was just huge, leading to a disaster.
Time will tell what happens. I expect that we will discuss this for quite some time as details become available. Please, anyone with deepwater oil experience please comment.
Finally, I mentioned last week that Dr. Isodore Rosenfeld completely botched his story about soft drinks last week on the Fox “News” Network. He actually started his bit this morning by issuing a clarification. But he got it wrong, again! He once again stated that phosphoric acid is added to some soft drinks to provide sparkle, which is completely wrong. It is the carbon dioxide that provides sparkle, and the phosphoric acid in some soft drinks renders them acidic (although the carbon dioxide also provides some acidity). If you take a bottle of seltzer and shake it up and make it go flat, it will taste just like plain water. If you do that to a phosphoric acid based soft drink, it will still taste sweet and sour, but not be fizzy. This guy is losing it! By the way, in certain parts of the United States, soft drinks are called “phosphates”.
Well, you have done it again! You have wasted many einsteins of perfectly good photons reading this piece of blogosphere pollution. And even though a certain Family Research Council (former) bigwig ‘fesses up to having intentions other than hiring the male escort as more that a porter during his trip when he reads me say this, I always learn much more than I could possibly hope to teach writing this series. Thus, please keep comments, questions, corrections (especially corrections), and other ideas flowing after the publish time. Remember, no science or technical issue is off topic here.
Warmest regards,
Doc
Crossposted at Dailykos.com

8 comments
Skip to comment form
Author
for an oily mess?
Warmest regards,
Doc
When you mentioned the toxins that will wash ashore, will there be toxins left in the unevaporated tar balls? To what extent will they harm the environment? – ShelleyShelley
I had no idea that BBQ Charcoal was carinogenic! If we do not come into physical contact with the tar balls, can we consider ourselves safe from those potential carcinogens?
I guess the labeling of “Wild Caught” won’t be considered as organic with this situation as it once was!
Author
Any and all questions are welcome.
Warmest regards,
Doc