( – promoted by buhdydharma )
Nuclear fusion is often proffered as the final solution to our energy needs. That well may be, but hardly anyone understands what it means, and almost no one, outside of physicists, knows how it relates to nuclear fission (the power source that we use now).
It all has to do with Dr. Einstein’s simple, but seminal equation, E = mc2. This means that mass can be converted to energy in a huge fashion. Let us take a kilogram of mass, any mass, and convert it to energy. Using the formula, and it has been proved over and over to be correct, one kilogram of mass (think of a big sirloin steak, for example) becomes a LOT of energy.
According to the equation, that kilogram of mass becomes thus:
E = (1 kg)(2.9979 x 108 m/s)2 = 8.99 x 1016 Joules
This is almost 90,000 billions of Joules. We are talking big energy. But it does happen quite like this. Only in matter-antimatter annihilation does all mass become energy.
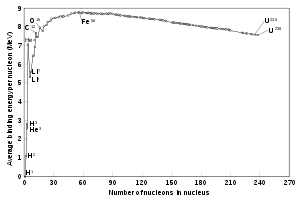
There are some concepts that are important. I already quoted the mass/energy equation, and it is important. The next thing that you need to know is the mass defect, also known as the packing fraction. This is the difference between the mass of the nuclei that either split (fission) or combine (fuse) to make new, more stable nuclei. This is the only important thing to remember. Everything else is just detail. Here is a chart that shows it. Please pay attention.
Note that on the left side, the curve is very steep. This plots the binding energy of nucleides versus their mass. Note that on the right side, the slope is very gradual, unlike on the left side. That means that uranium, for example, can fission to two or more lighter elements with a release of lots of energy. If you look closely, there is not a whole lot of difference in the energy curve, but that was enough for us to incinerate many thousands of people in 1945. I wish that we had not done that. My personal belief is that such a device, put well out to sea, would have made the same point. But that is just my personal belief. President Truman will have to deal with his decision if there is a deity.
UPDATE: due to a comment from Kossack maybeso in michigan, I have added this further explanation to the graph which just appeared.
On the x/-coordinate is the number of nucleons (the sum of protons and neutrons) in the nucleus. On the y/-coordinate is the binding energy of the nucleons, which, by E = mc2 is also directly proportional to the amount of mass that has been converted to energy to form a particular nucleus. That means that one approaches Fe-56 from either the left or the right side, energy is released as mass is converted to that energy.
It also means that you get lots more energy going from left to right, at least at first. That is why fusing four protons has the greatest energy yield, because the ordinary hydrogen nucleus, a bare proton, has zero binding energy because it is not bound to any neutron. Now, that means that all nuclei are thermodynamically unstable compared to Fe-56, but many nuclei both lighter and heavier are kinetically stable because the activation energy to get to Fe-56 is so high that the reaction does not occur at reasonable temperatures. Thus, helium is stable except in extreme circumstances, as are many lead isotopes. We will get into this more next time when we look at stellar fusion.
UPDATE II: commenter LookingUp has brought to my attention the the most stable nucleus is not Fe-56, but rather Nickel-62. The comment indicates that the difference is small, but real. It turns out that Fe-56 is usually the last nucleus to be formed in sun like stars because the mechanism (I suspect that the activation barrier is extremely high) to form Ni-62 is difficult. I apologize for the error.
Now, let us look at the graph. On the left side, it shows that fusing light nuclei into more massive ones gives much more energy. Indeed, that is true.
Normal hydrogen has only a proton for a nucleus, and thus has zero binding energy. Deuterium, the next hydrogen isotope (with a both a proton and a neutron) has some, but not a lot. Since the neutron is electrically neutral, it does not repel the proton, so no great binding energy is required for this nucleus.
The same goes for tritium, with one positive proton and two neutral neutrons. However, there in an imbalance there, because of the fundamental instability of neutrons. Tritium decays by beta decay in around 13 years into helium-3, which is stable.
These nuclei (and many, many more) all get fixed together because of interactions between particles that are even more fundamental than the proton and the neutron. Interestingly, the most stable nucleus is that of iron-56, and if you look at the graph again you will see that it is just at the top, barely. That means that normal stars, like our sun, can only produce elements up to that nucleus, or break down ones to that nucleus. This gets important in the next installment.
Let us consider the fusing of four protons into one helium nucleus, like the sun does.
That overall reaction can be summed up thusly:
4 protons (hydrogen nuclei) yield energy + (plus a few other products to be discussed next time)
In this reaction, four protons are fused into two protons and two neutrons in the form of a helium nucleus. The He-4 nucleus is anomalous in that is it extremely stable, because all four nucleons are in the ground state, the most stable state.
This reaction releases the greatest amount of energy know known, (2.48 x 1012 Joules per mole of helium produced, compare with 2.4183 x 105 Joules per mole of water formed when hydrogen is burnt in air, or around 20 billion times more energy) and ignites the sun. The sun has an advantage, since no living things are there, and it is so massive that it provides its own containment. In the core, the temperature is millions of degrees K, and the pressure is estimated to be so crushing that positive nuclei are overcome with energy of displacement (kinetic energy) that the electrostatic force is not enough to keep them apart. This is the activation energy for a reaction, and a reaction can be self-sustaining only if it releases enough energy in total to provide its own activation energy.
As an ordinary world example, think of a candle. Left alone, it will never burst into flame. However, if a match is put to the wick, enough energy is introduced to overcome the activation energy barrier so that the hydrocarbons in the wax begin to react with oxygen in the air. After the candle is alit, it continues to burn because the energy released from it is more than required to overcome the activation barrier for other hydrocarbon molecules.
Once that barrier is gone, the strong nuclear force becomes dominant. That force violently pulls the nuclei together, regardless of electrostatic charge, and they fuse to form heavier nuclides. However, in the sun this reaction is extremely slow because the weak nuclear force has to allow a quark to change in a proton to form a neutron and a positron. These are slow reactions. If the sun were our size, it would not even be at body temperature, even if this reaction could occur at low pressures and temperatures.
Next week we shall go through the proton-proton chain reaction that our sun uses to fuse four protons to one He-4 nucleus, but we need to finish the geeky physics behind nuclear energy first.
Now, let us try to understand energy from fission. That is when a single, heavy nucleus splits into two or more lighter ones. Look back at the chart, and remember that Fe-56 is the most stable nucleus. Thus, all of the light ones “want” to get there, and all of the heavy ones do, too. Now, the heavy ones can do this in several ways.
is not very common, since most of the nucleides that were ripe for it did so eons ago.
One way is spontaneous fission, meaning that a heavy nucleus would just spilt in two on its own. This is not very common now, since most of those did it long ago. We can synthesize nuclei that do it, but they do not last for very long. We do, however, use fission in current nuclear plants, but it is induced, not spontaneous in that we artificially enrich uranium-235 in fuel so that those nuclei will fission when bombarded by slow (thermal) neutrons. The U-235 splits into lighter fragments, thus going towards the left of the chart, since uranium is near the right edge.
For heavy nuclei, by far the most common way to try to get to iron is alpha decay. In that scheme, the unstable nucleus expels a He-4 nucleus (a very stable particle, that, when it captures two electrons, becomes helium gas) and loses both four units of mass and two positive nuclear charges. Thus, uranium-238 decays this way to thorium-234 by this scheme:
U-238 yields Th-234 + He-42 + energy
Thus, the uranium loses four mass units, and a little energy, becoming thorium-234. This isotope is unstable, and emits an electron to become Protoactinium/-234, a very rare element. On the other hand, there are lots Thorium-232, which decays by another alpha chain to Radium-228. The important thing to remember is that Thorium-232 has a half life of 14 billions of years, so it does not go away very fast. It can be turned into good fuel, and is not uncommon. It also makes our Coleman lantern mantles very bright when used as its oxide and heated with a hot gasoline flame, but that has everything to do with its chemical, and nothing to do with its radioactive, properties.
Now, there are some other considerations for fusion to work. One is that the probability of a reaction to occur is high enough once the activation barrier has been crossed that enough material reacts to be self-sustaining. This is called the cross section for the interaction. Another is, at least outside of the cores of massive stars, that only two nuclei have to interact at a time. Three-body collisions are extremely improbable except at phenomenally high pressures, so for all practical purposes only two-body processes are useful. The only candidates available for us on earth that fulfill all of the criteria are deuterium and tritium, one nucleus of each. In this system, the activation energy is low enough that it can be achieved, and the cross section is large enough that the reaction is self-sustaining. However, there is a problem.
Deuterium does occur naturally, in significant amounts, and can be had in ton quantities. A mole (about 4 grams) can be bought from Aldrich for $161, so it is not particularly pricy, especially considering how much energy can be had when it is fused. Tritium, on the other hand is tough.
Tritium does not occur naturally (except for few atoms that are created by cosmic rays, a negligible amount, so it has to be produced artificially. Also, as stated earlier, it is radioactive and decays with a half life of about 13 years, so it is sort of hard to hold on to once you have it. However, it can be made by bombarding the element lithium with neutrons, and that is how we make it. In the installment about terrestrial fusion we shall go into detail about this.
The problem with deuterium and tritium, both, is that they are gases. Since gaseous phase is the least dense form of matter, that sort of causes a problem because we need high densities to make fusion possible. This can be overcome, however, and this shall be discussed in a future installment.
The other real problem is that the energy yield of deuterium/tritium fusion yields only a little over a quarter of the energy of fusing four protons. This is not so much of a difficulty, I suppose, since the amounts of energy we are talking about are so high in the first place, but stars would not be very bright if they depended on this fusion pathway for their energy.
Next time we shall discuss stellar fusion (and how elements heavier than iron are made in the first place) and look at how our sun fuses protons, how heavier starts do it, and what happens when the hydrogen runs out (like it will in our sun in the distant future). After that, we shall look at terrestrial fusion, including the thermonuclear bomb, possible power production, and my speculations about the future of fusion for power production.
Well, you have done it again! You have wasted another perfectly good set of photons reading this, mmmm, stuff. And even though John Boehner stops spraying on his tan when he reads me say it, I always learn much more than I could possibly hope to teach writing this series, so keep questions, comments, corrections, and other thoughts coming. Remember, no technical or scientific subject is off topic here. Tips and recommendations are also always appreciated, and tell your friends about the series if they are interested at all in science, or even if they are not, because they might just get that way.
UPDATE III: well, folks, it is time to retire for the evening. I shall be back here for Review Period Monday night around 8 or 9 PM. Thanks for the support!
Warmest regards,
Doc
Crossposted at Daily Kos

5 comments
Skip to comment form
Author
for hot stuff?
Warmest regards,
Doc
featured an all day UFO sci-fi programming effort. Actually I should not say sci-fi but rather sci-fact in light of the viral dissemination of this information via the net.
http://www.enterprisemission.com/
http://www.google.com/#hl=en&q…
Maybe when hyper dimensional resonators are for sale on EBay.
http://www.google.com/#hl=en&q…
But I was there. I had some ideas about energy saving twisty lamps to explore. Our group though was a pain in the ass to the assholians of corporate pontificating compliance Seig Heil crap and we were terminated so you don’t have long lasting twisty lamps you have crap that is made by Chinese peasants who have less than one generation into it.
In 22 years of pure egalitarian exposure to science the one biggest conclusion that I can come to is that some people really are evil parasites which should be for the benefit of the entire universe eliminated for all time.
That turns out to be a spiritual thing.
Author
for the front page. I appreciate it very much. I shall return Monday evening around 8 or 9 PM Eastern time for Review Session.
Warmest regards,
Doc