(noon. – promoted by ek hornbeck)
Last time we talked about the history of the periodic table and some of the reasons behind why it “works”. We also took a look at the first three periods (rows), the very short first period, with only two elements, and the two short periods with eight elements each in them. We also grouped these elements into families (columns) that show similar chemical properties.
Now we shall look at Periods 4 and 5, the two long periods. These periods (and later ones) contain the transition metals. In the first three periods, chemical properties change radically from one element to the next as atomic number increases. For example, fluorine, the most chemically reactive element sits next to neon, which forms no known ground state chemical compounds.
Before we get started, we need a periodic table. Here is one that fits on the page.
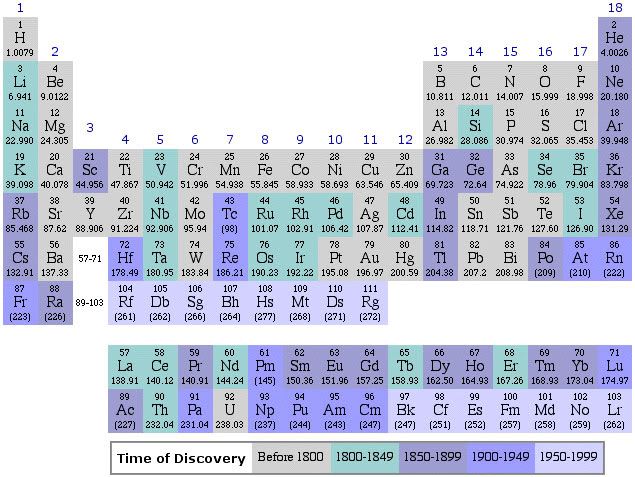
Here is a pretty nice interactive one, and you can see the electronic structure (amongst other properties) by clicking on the element. You may want to open it an a new window to follow along.
We have, last time, and will this time talk quite a bit about orbitals and suborbitals. Here are pictures of the spatial directions of electron density for the suborbitals that we have and will discuss. For each orbital, except the s orbital, the complete orbital is a superinposition of all of the suborbitals represented. The colors have to do with the phase of the electron density. That has little to do with atomic orbital electron filling, but becomes extremely important when molecular bonds are investigated, because only parts of orbitals of the same phase can form stable molecular bonds.
I have chosen the lowest energy orbital for each type. The higher shells have similar orbitals, but their spatial arrangement is a bit more complex. The exact meaning of the labels is not that important for this discussion, but they are the mathematical descriptors that physicists and chemists use for each suborbital.

1s
There is only one s suborbital, and it is the entire orbital. Any suborbital can have a maximum of two electrons, so an s orbital can have maximum of two electrons.

2pz

2px

2py
The three p suborbitals, superimposed, comprise the p orbital. Any suborbital can have a maximum of two electrons, so a p orbital can have maximum of six electrons.

3dz2
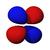
3dxz

3dyz
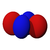
3dxy

3dx2-y2
The five d suborbitals, superimposed, comprise the d orbital. Any suborbital can have a maximum of two electrons, so a d orbital can have maximum of ten electrons.

4fz2

4fxz2

4fyz2
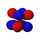
4fxyz

4fz(x2-y2)

4fx(x2-3y2)

4fy(3x2-y2)
The seven f suborbitals, superimposed, comprise the f orbital. Any suborbital can have a maximum of two electrons, so an f orbital can have maximum of 14 electrons.
The reason for these radical changes in chemical properties, as we saw, has to do with filling up orbitals, with a completely filled orbital being more stable than a partially filled one. Now we shall look at another topic that is critical in understanding the chemistry of the elements: only the outermost electrons have a significant effect on chemical properties. For example, lithium and sodium behave similarly chemically, but sodium has both a filled K shell and a filled L shell. The M shell has only one electron, just like lithium has its L shell with only one electron. In other words, electrons inside of the outermost, unfilled, shell have little effect on the chemistry of the element.
Starting with Period 4, the N shell is the outermost shell. Now remember what we determined last time: every time the period increases by one, the number of orbitals within that shell also increases by one. Thus, Period 1 has only one orbital, the s orbital. Period 2 has the s orbital and a p orbital, and Period 3 has the s and p orbitals and also a d orbital. Two electrons fill an s orbital, six fill a p orbital, and ten fill a d orbital. You might stop me and say, “But, Doc, Period 3 has d orbitals, but only the s and p orbitals are filled! What gives?”
That is a very good question. It turns out, because of spatial arrangement, the 3d orbital is very slightly higher in energy than the 4s orbital, but considerably lower in energy than the 4p orbital. This is because on average, the distance between the nucleus and the maximum electron density of the 3d orbital is slightly greater than that for the 4s orbital, and considerably lower in energy than the 4p orbital. All s orbitals have only a single suborbital, and two electrons fill any suborbital. All p orbitals have three suborbitals, so six electrons fill a p orbital. All d orbitals have five suborbitals, so ten electrons are required to fill a d orbital.
This explains why Period 4 is the first long period, rather than Period 3. After the p orbital in Period 3 is filled, the next lower energy orbital is 4s. Thus, after the inert gas argon, the last member of Period 3, the alkali metal potassium, element 19, has a single electron in the 4s orbital. Calcium, element 20, is next with two electrons in the 4s orbital. Now something interesting happens. Instead of filling the 4p orbital next, the lower energy 3d orbitals begin to be filled. Element 21, scandium, adds an electron to the 3d orbital, leaving two electrons in the 4s one. Now, instead of the outermost orbital changing number of electrons, an inner one does. The chemistry difference between calcium and scandium is much less than that between magnesium and aluminum, so there is a slow transition in properties from Group 2a and Group 3a in Period 4 than their is in Period 3, hence the name transition metals.
As electrons are added to the 3d orbital, the energetics change from those of unoccupied orbitals, so the electrons are not added in anywhere near a uniform manner. For example, titanium and vanadium, elements 22 and 23, add electrons to the 3d orbital one at a time, but chromium, element 24, has a bizarre electronic configuration. In chromium, one electron is added to the 3d orbital, and one of the 4s electrons falls to the 3d orbital. Actually, this is not as bizarre as it sounds. As electrons are added to the 3d orbitals, remember that they are added to separate suborbitals first, and only begin pairing after all the suborbitals are half full (with one electron). Thus scandium has one suborbital with one electron in it, titanium has two with one electron each in them, vanadium three with one electron each, and then chromium puts two more electrons into the d orbital, half filling all of the suborbitals. This is a ramification of the Aufbau (building-up) principle, and the energetics favor the 3d orbitals to all be half filled, even at the cost of half-filling the 4s orbital. The next element, manganese, adds an electron to the 4s orbital, leaving the 3d one with all suborbitals half occupied.
Since the 4p orbital is considerably higher in energy than the 3d one, it is not utilized until the 3d orbital is completely filled. Hence iron, element 26, adds an electron to one of the half occupied 3d orbitals, and that trend continues through element 28, nickel. Element 29, copper, adds an electron to the 3d orbital and abstracts one from the 4s orbital, thus completely filling the N shell. This is an especially stable configuration and pays for the energy cost of having an unpaired 4s electron. Element 30, zinc, adds its last electron to the 4s orbital, and this ends the transitions metals in Period 4. The rest of the elements add electrons to the 4p orbitals just like their relatives just above them in the Periodic tTable, and instead of a gradual transition of properties, the major chemistry changes seen in the members of Period 3 are seen.
This is a beautifully graphical way to show that the 3d orbital requires 10 electrons to fill it, because Period 3 has only eight elements, whilst Period 4 has 18. I realize that this has been particularly geeky, but the transition metals are important. Some of our most essential structural materials are transition metals, such as titanium and iron. Copper is of extreme value for electrical and plumbing applications, and zinc is used in huge quantities to protect iron from corrosion. If one looks at Period 5, a very similar trend is seen, because the 4d orbitals from Period 4 are very slightly higher in energy than the 5s orbital and considerably lower in energy than the 5p orbitals. Some of the details of how electrons are filled into the 4d orbital are a little different than the way that the 3d orbitals are filled, but the trends are very similar.
Now, the N shell has s, p, d, and f (unoccupied) orbitals, just like the M shell had the unoccupied d orbital in addition to the s and p orbitals. In exactly the same fashion that the 3d orbitals were not used in Period 3, the 4f orbitals are not filled in Period 5 because they are too high in energy. Thus, Period 5 is the same length as Period 4, with 18 elements, ten of which are transition metals.
This means that the first 54 elements in the periodic table use only s, p, and d orbitals for their electrons, although there are unused f orbitals that are too high in energy to compete with the lower energy orbitals. These f orbitals will come into play when we look at the lanthanide (rare earth) metals and the actinide (which contain uranium and plutonium) metals. These elements use f orbitals in lower shells and form another series just like the transition metals in Periods 6 and 7, respectively. These f orbitals have even less influence on chemical properties of their respective elements than the d orbitals do, and the chemistry of the lanthanides and actinides is quite difficult with respect to separating one element from the other. Modern ion exchange methods revolutionized the separation of these elements from each other, and it is possible to provide them in high purity in useful quantities except for the ones that are too rare or too intensely radioactive to be of use.
All known elements use only up through the f orbital for fitting electrons. At present, the element with the highest atomic number is element 118, and f orbitals are sufficient for its electrons. However, from theory, element 121, if it is ever discovered, should begin using g orbitals, making Period 8 an extremely long period, with 9 different g suborbitals, in addition to the others mentioned earlier in known elements. That period would then contain 50 elements, almost half the number of known elements today through Period 7. It is unlikely that Period 8 will ever be filled with known elements.
Well, you have done it again. You have wasted another perfectly good batch of photons reading this poor post. And although William Kristol becomes a dove when he reads me say it, I always learn much more than I could possibly hope to teach writhing this series. Please keep those comments, questions, corrections, and other information coming. Remember, no scientific or technology issue is off topic here.
Warmest regards,
Doc
Crossposted at Dailykos.com

6 comments
Skip to comment form
Author
pretty shapes and colors?
Warmest regards,
Doc
I especially like the interactive periodic table. Great teaching tool.
Wonderful teaching tool. Thanks, Doc.