( – promoted by buhdydharma )
This is the second of two installments on non-nutritive sweeteners. This time we will talk about one very ancient one, the new ultra sweet aspartame analogue, and a couple of natural products, one of which is gaining popularity these days.
Non-nutritive sweeteners probably have value in managing conditions such as diabetes and obesity where caloric intake, particularly from simple carbohydrates, needs to be restricted. However, these materials are not panaceas and other dietary measures are essential to control either of those conditions. Some studies also are consistent with the premise that the mere sensation of sweetness can cause a rise in blood glucose in non-diabetic people, thus making these agents act like sugar even though they contain no calories. These interpretations are controversial, though.
Other than sugar (and honey, etc.) itself and a few natural materials used in very limited areas, the oldest sweetener is lead acetate (still called “sugar of lead”). This material was very popular with the Romans. They would boil down grape juice to concentrate the sugar in it to use as a sweetener. The problem was that they often used lead boilers for this process, and likely more often than not the grape juice has begun to sour, forming vinegar (acetic acid). This would react with the lead vessels, forming lead acetate (one of the very few soluble lead salts). Due to the sweetening power of the lead acetate, it is likely that the sweetener made in lead vessels had more sweetening power than an equivalent material made in earthen or copper vessels.
In any event, lead acetate is certainly not a good idea for use as a sweetener, since it is both acutely and chronically toxic. I am not aware of any country where lead acetate is approved as a sweetener, and that practice is exceedingly hazardous. This has been included just as a historical note. People have been looking for sweeteners for a long, long time.
We covered aspartame last time, and I was aware at the time that a new analogue of it was in the works, and last week I saw the first product (in my experience) that contains it on the shelf at my local Kroger store. It was one of those flavored water drinks (I do not remember the brand) in one liter plastic bottles. That sweetener is called neotame, and the NutraSweet company developed it several years ago. FDA approved it in 2002, but I had never seen anything with it in it until last week. Structurally, it is very similar to aspartame. Here are the structural formulae of aspartame and neotame for comparison:
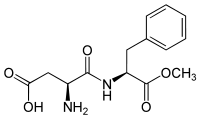
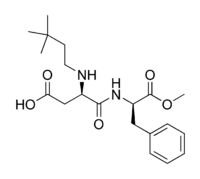
Aspartame Neotame
Neotame is much sweeter than aspartame. Where aspartame is around 300 times sweeter than sugar, neotame is somewhere around 10,000 times sweeter. (Absolute comparisons are difficult because of subjective complications and nonlinearity of the relationships with varying concentrations). The name “neotame” is interesting. “Neo” is a combining form meaning “new”, and this is a relatively new sweetener. In addition, if you look at the structural formula you will see a 3,3-dimethylbutyl group attached to the nitrogen on the aspartic acid (just left of center in this diagram). Another name for a 3,3-dimethylpropyl group (one carbon shorter than the dimethylbutyl one) is “neopentyl”. I do not know if they were thinking of that when they named it or not.
Neotame is more heat stable than aspartame because of steric effects of the 3,3-dimethylbutyl gruop. When aspartame is heated in water, a reaction occurs that adds a water between the aspartic acid the phenylalanine halves of the molecule. The 3,3-dimethylbutyl group is very large, in molecular terms, and blocks the attack by water to a rather large extent, rendering the molecule more stable to heat. It is also not metabolized to phenylalanine, so there is not an issue with phenylketonuria. The methyl group on the phenylalanine part still gets hydrolyzed to methanol, so the thermal stability is not absolute. Neotame is so much sweeter than aspartame (30 times sweeter, give or take), than any concern over methanol toxicity (and I think that this is a red herring for aspartame) is reduced by a factor of 30 for neotame.
I have not tasted any of the products sweetened with neotame, so I can not attest to any aftertaste or lack of aftertaste. Anyone with experience is encouraged to describe her or his experiences.
A sweetener used in Europe to some extent and in Japan to a larger extent is glycyrrhizin. This is a sweet (somewhere around 3 times sweeter than sugar, comparable to cyclamate) material extracted from the root of the licorice plant (Glycyrrhiza glabra) from south America. Glycyrrhizin is not approved in the United States as a sweetener, but licorice root extract is used as a flavoring.
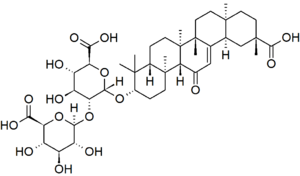
Glycyrrhizin
Obviously this a rather complex molecule, and sure enough, there are some health concerns associated with it. In fairly small doses it interferes with steroid metabolism, suppressing cortisol and agonizing the mineralcorticosteorids. This can have several effects, including reduced ability to cope with physical stress (cortisol is one of the stress hormomes) and causing fluid retention and electrolyte imbalance. Concomitant with fluid retention, blood pressure is often elevated. These are some serious medical concerns. In the UK, where it is approved, officials recommend using as a maximum 100 milligrams per day, and the Japanese guideline is 200 mg. At 30 times sweeter than sugar, that comes to about six grams of the sweetness supplied by sugar (using the 200 mg guideline). That is about 1.5 teaspoons of sugar, or around 22 calories. This is hardly a significant impact on total caloric intake.
The subjective sweetness of glycyrrhizin is said to be different than sugar, with a slower onset and longer duration. I do not see much utility in glycyrrhizin as a non-nutritive sweetener, and the health effects are significant enough to be of concern.
Another natural product that has been increasing in popularity in the United States is Stevia, the leaf of Stevia rebaudiana, a south American plant in the aster family. Stevia is available as the whole leaf, extracts of the leaf, and as live plants. FDA banned Stevia in 1991, declaring it unsafe, even though no study had ever shown it to be unsafe. The dietary supplement law enacted in 1994 caused FDA to allow Stevia as a dietary supplement, but not as a sweetener, although that is pretty much the only use for it.

Stevia rebaudiana
Well, money talks. The Coca-Cola Company, partnering with Cargill, developed a sweetener called Truvia and at about the same time Pepsi-Cola, partnering with Merisant (which used to be part of Monsanto but spun off) developed PureVia. FDA wasted little time in approving both of those, putting them on the coveted GRAS (generally recognized as safe) list, meaning that there is essentially no restriction on their use. They have been thus approved since 2008.
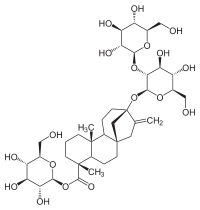
Stevioside
Obviously, stevioside is a complex molecule. It is the most abonndant sweet component in crude Stevia and crude extract and is around 300 times sweeter than sucrose. Another component, a little less abundant but around 400 times sweeter than sucrose is rebaudioside A. The only difference is tht rebaudioside A has an extra glucose molecule at the top of this diagram (I could not find a clear diagram of rebaudioside A). It not only is sweeter, but is also said to be less bitter than stevioside, and is the material used both in Truvia and PureVia. Because these materials are so sweet, they have to be cut with fillers so that measurment is possible with standard kitchen utensils.
As I said, money talks. Whilst FDA approved rebaudioside A for general use, the stevia plant and crude extract is still approved only as a dietary supplement. That is sort of like saying that cane sugar is OK as a food, but cane syrup is only to be used as a dietary supplement. What that means is that the big boys have what is essentially a monopoly on Stevia, since their patented products are the only ones on the GRAS list (an so can be added to any food product), whilst the unpatentable plant itself is available only as a supplement, so you have to buy it separately and add it yourself. Interestingly, these FDA actions were taken in the waning days of the Bush administration. Because of these shenanigans, I refuse to buy either Truvia or PureVia.
It is astonishing to me that FDA went from a complete ban in 1991 to GRAS listing for the commercial products in 2008. That is how big business, combined with a very pliant regulatory body, does things. I do not think that the products are hazardous, but the process sure is. I recall similar situations involving Big Banking and Wall Street. That administration was the gift that keeps on taking.
There have been many, many studies performed on Stevia, its extracts, and the purified sweet principles and none have shown any negative health effects. The food and medical community have pretty much come to a consensus that the herb and the components are not harmful and actually may have some beneficial effects, such as increasing insulin sensitivity (which would be a real benefit to Type II diabetics). I just do not like the way that the politics played out in the entire issue.
Well, you have done it again. You have wasted another perfectly good batch of photons reading this poor effort at an essay. And even though Van Jones decides not to resign every time he hears me say it, I learn much more than I could ever possibly hope to teach whilst writing these essays, so keep those questions, comments, corrections, and other topics coming.
I promised a commenter last time to look for the glycemic impact of maltitol, one of the sugar alcohols. Alas, I was not able to find a good comparison betwixt it and the other sugar alcohols. If anyone has a link, please provide it and I will update this installment.
Warmest regards,
Doc
Crossposted at Dailykos.com

9 comments
Skip to comment form
Author
for more sweet stuff?
Warmest regards,
Doc
Author
Thanks, Buhdy! I very much appreciate it.
Warmest regards,
Doc
http://sweetleaf.com/
No connections to Coke or Pepsi. No padding with dextrose or other questionable ingredients. They have a deal where you can get two free samples to see whether you will like the taste. You can order it over the net or get it in local health food stores at the same price.
The extract is more concentrated than sugar – I use about 1/2 tsp per 16oz cup it to sweeten my tea daily, and I go through a 4oz jar in about 8 weeks.
Ask yourself why the FDA would completely ban, then discourage a safe, natural, plant-based sweetener but push through a product that breaks down into methanol and formaldehyde when heated. Hint: the health care “industry” needs to stay in “business” as much as the soda pop “industry” does.
Ask yourself why Nutrasweet STILL sells aspartame in packets meant to be used in coffee and tea, which are served hot, despite the fact that it is becoming common knowledge that it’s not supposed to be used in heated food or drink. When ignorant people make the decision to put the stuff into their own hot drinks, they assume the legal responsibility for that decision regardless. This is a classic example of a corporation preying on the ignorance and trust of the American public, and being allowed to do so by a complicit government.
Google Donald Rumsfeld’s involvement with getting aspartame ramrodded through the FDA, the info is all over the internet now.
Many years ago my father made sure to leave Sinclair Lewis’ novel Arrowsmith laying around where I would find and read it. I never forgot the lessons embedded in that story, which was written in 1925. Nothing has changed in the attitudes of the corporations which support the health care “industry” – indeed, they’ve only gotten worse.