(9 am. – promoted by ek hornbeck)
There is a lot of attention regarding the topic of trans fats, but hardly anyone outside of chemists and biochemists really understands what a trans fat actually is. This evening we will discuss what they are, whence they come, and some health aspects of them.
This is a controversial subject (not as controversial as high fructose corn sweetener), in that the medical community is not completely in unison with the interpretation of the data from studies. However, the case is more clear than with high fructose corn sweetener.
First, some housekeeping. I STILL have not bought cigarettes since March, but still continue to roll the Prince Albert, but I have cut down somewhat. More progress to go.
Although this statement might run some readers away from here, we need to look at fundamental chemistry for a few minutes. The concept of cis and trans (modern usage prefers Z (the German for zusammen, “together”) and E (the German for entgegen, “apart”) has to do with geometry on a molecular level. This is critically important to understand, so we will start with the simplest organic molecule that displays this behavior.
Behold 2-butene, (trivial name is 2-butylene), the simplest organic molecule that display this difference. Butene consists of a family of molecules with the formula C4H8 AND a double bond somewhere in the carbon chain. There are two ways of stringing those atoms together (not counting making a four-membered ring, with no double bond): either 1-butene or 2-butene. The numbers refer to where the double bond is. There is only one possible 1-butene, but two possible 2-butenes. Here are the pictures of them, and please pay attention. This is important for understanding the entire issue.
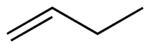
1-Butene
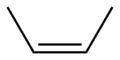
cis-2-Butene (aka Z-2-Butene)
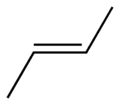
trans-2-Butene (aka E-2-Butene)
You can see that in the 2-butene cases that there are two possible arrangements for the larger (methyl groups rather than hydrogen atoms) groups around the double bond (by the way, the geometry of that bond is 120 degrees in a single plane). Because of quantum mechanical reasons, and also common sense, the more stable (lower energy) form of 2-butene has the larger groups facing away from each other (the trans form). The cis form, with the two groups closer together, is less stable (higher energy). This is easily demonstrated by burning samples of each material and recording the amount of heat released. The trans one produces less heat than the cis one does, because it has less energy to begin with, making it, in the thermodynamic sense, more stable. In other words, the trans one is lower in energy than the cis one, and, at ambient temperatures, is thus favored.
OK, enough with thermodynamics for now. We need to know what a fat is. This is pretty easy. A fat is, by definition, one glycerol (aka glycerin) molecule combined with three fatty acid molecules. Here is a picture of glycerol:
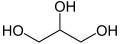
Glycerol
Note that there are three -OH groups along the carbon chain. Glycerol is an alcohol with three active -OH sites, called a trihydroxy (old term, tribasic) alcohol. (Ethanol is a monohydroxy (monobasic) alcohol, with only one -OH group. Here is a picture of it for comparison:
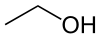
Ethanol
To form a fat, three fatty acids must bond with the glycerol. Fatty acids are very common in nature, and are long chain (12 to 18 carbons long, usually) materials with a carboxyalic acid group (<b>-COOH) at one end. During the reaction with glycerol, one water molecule is eliminated for each -OH on the glycerol, making a larger molecule. When all three active -OH positions on the glycerol have been reacted, we have a fat. The technical term for a fat is triglyceride, and those of who who watch your blood lipid levels will recognize that as one of the three things to monitor. (Those of you who read food labels will recall that in many foods monoglycerides and diglycerides are on the label. Those are like fats, but only one or two of the active -OH groups on the glycerol have been bonded to a fatty acid. We can talk about why they are put in food in the comments.
The chemistry lesson is almost over, but there is one more key concept that is important to know. Fatty acids sometimes have double bonds, just like butene. Not all of them do. For example, saturated fats contain three fatty acid molecules bonded to glycerol, and none of the fatty acids have carbon-carbon double bonds. Stearic acid, common in beef fat, has this formula. Note that the only double bond is between carbon and oxygen in the acid group, so it is a saturated fat.
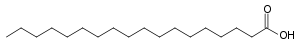
Stearic acid
However, many fatty acids DO have a double carbon-carbon bond, and some have more than one. The predominant fatty acid in olive oil is the monounsaturated (and very healthy) one oleic acid:

Oleic acid
It is just like stearic acid, except there is the double bond in the carbon skeleton. Note that the long chains are on the same side of the double bond.
When olive oil is hydrogenated, some of the oleic is transformed to elaidic acid, which has the double bond in the same place, but now the long carbon chains are on opposite sides of the double bond. The rest of the oleic acid is transformed to stearic acid by absorbing a hydrogen molecule.
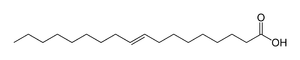
Elaidic acid
The lesson to carry away is that all of these acids are cis across every double carbon-carbon bond. This is critical. In nature, except for a few per cent of unsaturated fatty acids in animal products, all of the bonds are cis. With vegetable oils, all carbon-carbon double bonds are cis. By the way, these monounsaturated (one carbon-carbon double bond) and polyunsaturated (two or more carbon-carbon double bonds) fats have been shown to be very good for us, when used to replace saturated fats. Olive oil is pretty much accepted to be heart protective.
But unsaturated fats have one big problem (and a couple of small ones). The big problem is that they are not as stable as saturated fats, and react with atmospheric oxygen to become rancid. This occurs at the carbon-carbon double bonds, and makes the shelf life of polyunsaturated fats low, especially at room temperature, exposed to the air (like in a box of cookies, for example). Unsaturated fats also tend to have lower melt points than saturated ones, making them hard to use for making pastries, since solid fats are needed to make layers with the flour. In the extreme, like linseed oil, there are three double bonds in a lot of the fatty acids making up the fat (a little over 50% of alpha-linolenic acid), making it very reactive with oxygen. This is why linseed oil is used in oil based paint, because the reaction with atmospheric oxygen causes a hard film to form, thus “drying” the paint. Here is a picture of a hypothetical linseed oil molecule. The glycerol is on the left, and the fatty acids on the right.
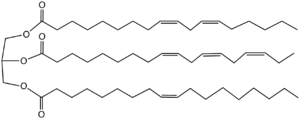
Linseed oil
Enter Crisco, the first hydrogenated vegetable oil. It was found that some of those carbon-carbon double bonds could be eliminated by subjecting oil (originally cottonseed oil) to high pressures in a hydrogen atmosphere with a nickel catalyst suspended in the oil. Some of the carbon-carbon double bonds would absorb hydrogen (all will if carried out long enough, and with enough hydrogen) and become saturated. The product was a solid fat at room temperature with much better working and keeping properties than the original oil. The nickel is filtered off and regenerated for the next batch. (As a professional chemist, I am not aware of any purification process that has completely unit efficiency, but that is another story).
So now we have a fat that is stable, easy to use, and bland (unlike lard, the favored fat for bread and pastry up until then, which has a bit of characteristic taste and is not Kosher, whilst Crisco is Kosher). Actually, this was a partially hydrogenated vegetable oil. Not all of the double bonds are saturated, and this is the problem. It turns out that if all of the carbon-carbon bonds are hydrogenated, the resulting fat is too hard and has too high a melt point to be very useful, becoming more like tallow. The partially hydrogentated fats found a balance between being stable enough for long shelf life and soft enough to be easy to use.
So far, so good. But the hydrogenation process has a downside. It turns out that for hydrogen to add across a carbon-carbon double bond, that bond has to be weakened. This is what the nickel catalyst does. It forms a charge complex with the double bond, allowing free rotation, and whilst the bond is at an opportune position, the hydrogen adda. The nickel comes and goes from time to time on all of the double bonds, adding and being released. But if not enough hydrogen is added to the mix to saturate all of the double bonds, the nickel is released after the normally cis bond becomes locked into the more thermodynamically stable trans one. Those trans fatty acids have no bad taste and are a bit more stable than the cis ones, so the shortening produced has excellent cooking and keeping properties. Typically, around half of the remaining unsaturated fats become trans after hydrogenation.
End of chemistry lesson. Thank you for bearing with me. It was important to develop this to allow you to understand why this is done, and how the chemistry works.
The problem is that our enzyme systems are not evolved to metabolize trans fatty acids in any large amount. The only natural source of them is a low concentration in animal products, and we can handle that few per cent. As a matter of fact, that particular trans fatty acid, vaccenic acid, is dealt with quite effectively by our enzyme systems, being transformed to rumenic acid, which is cis, trans and is implicated as having anti-cancer properties.
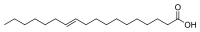
Vaccenic acid
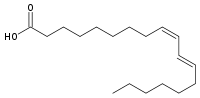
Rumenic acid
However, when, in the early 20th century, saturated fats and cis fats were suddenly replaced with the more convenient (and profitable) partially hydrogenated fats, with a very high content of trans fatty acids, our bodies could not cope very well. Whilst the cis ones, and even the saturated ones, are metabolized relatively efficiently, the trans ones sort of short out the enzyme systems. Trans fats have been implicated in everything from Alzheimer’s disease to obesity, but the strongest evidence to date makes it pretty clear that trans fats are certainly associated with heart disease. Here are some of the data.
The LDL/HDL (“bad” cholesterol/”good” cholesterol) ratio, an important measure of probability heart disease, has been shown to be worsened by consumption of both saturated fats and trans fats, but trans fats not only raise the level of LDL, like saturated fats do, but also also lower the level of HDL, unlike saturated fats. This double whammy makes them less healthful than lard or butter.
Another study indicates the the level of CRP (C-reactive protein), a marker for inflammation in the body, is much higher in folks who eat the most trans fats compared with those who eat the least. CRP is strongly associated with heart disease.
An extremely large (population 120,000) study of nurses over decades has shown that replacing carbohydrate calories with trans fats almost doubles the risk of heart disease for each 2% of carbohydrate calories replaced. Saturated fat requires 15% replacement of carbohydrate calories to show the same effect, making trans fat 7.5 times worse that saturated fat. Additionally, replacing trans fat with healthy unsaturated fat at 2% of total unsaturated fat intake reduces the risk of heart disease by over half, while it takes 5% replacement of saturated fat by healthy unsaturated fat to almost half the risk for heart disease.
This is pretty clear evidence, and it is a good thing that FDA has mandated trans fat content on foods, right? Well, no so much. For one thing, bulk foods (like those supplied to schools, hospitals, restaurants, and the like are exempt from those labeling requirements. For another thing, labeling requirements are such that if a single serving of a particular product contains less that half a gram of trans fat, it can be called “zero”. Now, assuming that man food products that one eats has that half gram, it is easy to eat several grams of trans fat per day and not even know it. Big Food pushed very hard for this technicality, and I think that it needs to be corrected.
One product is being advertised on the TeeVee that points out this fact. It is Smart Balance spread, a butter substitute. I do not own any stock in the company, but think that folks trying to do the right thing get some recognition. Their adverts go on to say that the only way to determine if there is trans fat in a product is to look at the ingredient statement and look for the term “partially hydrogenated vegetable oil”. That is pretty much correct, although some of the newer hydrogenation processes produce less that the traditional ones do, but these are not much used yet. Crisco claims to be less than one gram per tablespoon (it used to be 1.5), but that is still a lot.
Sometimes you will see a product that has “completely hydrogenated vegetable oil”. This material does not contain trans fat, but it fully saturated, like beef tallow almost is.
Personally, I eat hardly any trans fat, except for the unknowns that you get in the restaurant. I have used butter rather than margarine for decades, and make bread and pastries with lard rather than vegetable shortening. I always look at labels and make sure that I do not buy anything that has partially hydrogenated vegetable oil in it unless it is something that I really need and for which no substitute is available. It is not that hard to do, and can save you a lot of trans fat even though the label may say “zero grams trans fat per serving”.
Well, you have done it again. You have wasted another perfectly good batch of electrons reading this poor excuse for an essay. And even though Rush Limbaugh says that he hopes that President Obama succeeds when he reads me say it, I always learn much more writing these posts than I could possibly hope to teach. So, keep those questions, comments, corrections, and other remarks coming. Remember, no science or technical issue is off topic in the comments.
Warmest regards,
Doc
Crossposted at Dailykos.com

8 comments
Skip to comment form
Author
for healthy fats?
Warmest regards,
Doc
cringes at the American diet. I don’t think she even knows what margarine tastes like. Crisco is an anathema, not even for pastry dough. She has wine with her meals, eats nothing deep fried, uses as much fresh vegetables & fruits that are available, as she can. She has her wine every day and at night her vodka martini with a twist in the evening. The lady has no medical problems and has never taken as much as an aspirin in her life. I cook pretty much as she does and none of us have high cholesterol or blood pressure.
Another great diary , Doc. You are sounding well. Last night was my last night in the ER where I have worked for 30 years. I decided that the 12 to 16 hour days 4 to 5 times a week was more of a grind than I am willing to continue doing. I am in a fortunate position to do this. Emergency Medicine will always be my passion and I will continue to work with the International NGO that I have been with for 12 years.