(9 pm. – promoted by ek hornbeck)
This seems to be a topical topic (please forgive the confoundment of words) because of the controversial claims that the purported “Doctor Oz” gave last week about arsenic in apple juice. I shall give a couple of links later about that, but shall first describe the element in a Geeky way.
Then I shall dismember “Dr. Oz’s” credibility. Fair enough?
Before we get started, know that arsenic is all around us, at higher or lower concentrations, depending on where we live. I shall get into that a bit as well. The important thing to come away with from this post is that arsenic is almost (but not ALWAYS) a bad thing to ingest or to have for an injection. On the other hand, it likely is allowing us to communicate via the Internet as we read and speak.
Are you ready to start? I am!
Arsenic is with us. That is just a fact. Whether from drinking water in places where it is more common that it should be, to contaminants in foodstuffs, to pesticides, it is with us. It has an atomic number (henceforth called Z) of 33. Arsenic has only one stable isotope, which is not unusual for elements with an odd Z because of quantum mechanical considerations. Ar-75 is that isotope, with 42 neutrons, making it an odd/even element.
Since it is not a first or even second row element, it has some unusual quantum mechanical properties as well. It it is directly below phosphorous in the Periodic Table, and since it many of the same chemistries that phosphorous has, some of its toxicity can be explained on the basis that it interferes with phosphorous metabolism in multicellular life.
You would be surprised how much arsenic is used. Currently, around 2500 metric tons of arsenic are used annually in the United States, for several diverse products. For example, it is added to lead to harden it for use in lead acid batteries and in lead shot. It is also used in some copper alloys for the same reason. It is also extensively used in the semiconductor field as a dopant for silicon, providing the p type of semiconductors. The compound gallium arsenide is also an important semiconductor material with somewhat unique properties.
Not nearly as much arsenic is used now as was just a few years ago. In 2002, for example, about 18,000 metric tons of arsenic were used to produce the wood preservative CCA, chromated copper arsenite. This accounted for around 90% of US usage. CCA treated lumber was phased out starting in 2004, and in 2007 only around 2600 metric tons were used for that out of a total consumption of 5200 tons. Today, consumption is around 2500 tons for all purposes, and it is no longer used for wood preservation here.
It is, however, used in some agricultural settings yet. An organic arsenic compound, disodium methyl arsenate, is used on golf courses to control earthworms! Can’t have earthworms on the course! Up until around 1960, lead arsenate was extensively used to control insects on fruit trees, especially apples, and since arsenic is persistent in the environment, trees are still taking arsenic up through their root systems. We shall get back to this later.
Arsenic has been used as a poison for ages, and there are any number of references to it in the popular culture and the scientific literature. However, arsenic has been used in (and still is being) medicine. The first really effective anti-syphilis agent, arsphenamine, was first used in 1909. It was replaced by the more effective neosalvarsan in 1912 and that remained the first line therapeutic agent for syphilis until antibiotics were developed during and after World War II. There is no clinical indication for them any more. However, arsenic trioxide, the prototypical poison, is currently approved for treatment of acute promyelocytic leukemia that does not respond to less toxic agents. Obviously, dosage has to be carefully controlled so not to kill the patient. Still, nerve damage can and does occur.
Did you know that poultry producers intentionally feed arsenic to birds? Yes, indeed they do. The compound roxarsone, an organic arsenical, is used in about 70% of the poultry produced in the US as a growth promotant. Think about that the next time you sit down to a chicken dinner! Organic poultry can not be labeled as such if arsenicals are used. One important quality control check in the poultry industry is the quantitative analysis for arsenic in tissue samples to minimize the amount of arsenic in the product being marketed.
Let us return to CCA treated wood for a moment. Even though this material has not been used in the US for years, LOTS of it is still in service because CCA works really well. Interestingly, CCA was the replacement for “penta”, or pentachlorophenol, another very effective wood preservative, banned because because the manufacturing process left it with high concentrations of dioxin, another known carcinogen. In any event, arsenic from CCA treated wood leaches into the soil, so there are lots of playgrounds with CCA treated (or formerly had CCA treated) playground equipment that have high enough arsenic levels to be of concern. Another problem with CCA treated timber is disposal. The arsenic leaches out if landfilled, and in burnt some of the arsenic is released in the smoke (NEVER stand in the smoke from CCA treated lumber fires), and the residual ash is also extremely high in arsenic. It is estimated that 20 grams of some ash from heavily treated timber approaches the fatal human dose.
There are no sole purpose arsenic production facilities in the US any more. US arsenic production is from byproducts from other smelting operations, notably for lead, zinc, and copper. However, this meagre supply is not enough for domestic demand, so in 2006 the US imported around 10,000 tons, but we exported around 5500 tons as refined metal. Thus, our domestic consumption is around 4500 to 5000 tons annually.
China is the world’s largest producer, providing around 50% of the world demand of around 60,000 metric tons.
Now we go to Dr. Oz. These figures are from his website. Oz had 36 samples of juice analyzed for total arsenic, and ten of those samples came in at over the drinking water standard of 10 parts per billion (ppb). Here is a direct quote from his site:
Of these, 10 samples came back higher than the arsenic limit allowed in drinking water.
Note: Lab results standard deviation is +/- 20%
Minute Maid Apple Juice
Lowest Sample for Arsenic: 2 parts per billionHighest Sample for Arsenic: 3 parts per billion
Apple and Eve Apple Juice
Lowest Sample for Arsenic: 3 parts per billion
Highest Sample for Arsenic: 11 parts per billion
Mott’s
Lowest Sample for Arsenic: 4 parts per billion
Highest Sample for Arsenic: 16 parts per billion
Juicy Juice
Lowest Sample for Arsenic: 2 parts per billion
Highest Sample for Arsenic: 22 parts per billion
Gerber
Lowest Sample for Arsenic: 3 parts per billion
Highest Sample for Arsenic: 36 parts per billion
Now, you can see a problem right away. First, if the figures on his site are accurate, Minute Maid’s highest reading was only 3 ppb, less than one third of the drinking water standard. Second, of those 10 samples, only four show over the drinking water standard, one by only 1 ppb. Thus, out of 36 samples, only four were over the drinking water standard. Thus, the 10 out of 36, or nearly 28% of the samples tested being over the drinking water standard is just incorrect. Actually, only four (and possibly three, if you take the 20% standard deviation into account, were over the drinking water standard, or just over 11% (or just over 8% if that one 11 ppb one was on the high side of the standard deviation). Eight per cent is not nearly as alarming as 28%, and does not make for good TeeVee.
Furthermore, comparison of apple juice with drinking water standards is without merit. First of all, if children are drinking apple juice like water, there is a more fundamental problem than arsenic. Whilst apple juice has some vitamin C and potassium, it is loaded with sugar, to the tune of 130 calories per eight ounce serving! In comparison, my jug of Dr. Pepper has only 100 calories per eight ounce serving. My neighbor’s Mellow Yellow has 116 calories per eight ounce serving. Thus, apple juice has more calories than soda, and for that reason alone should be given only in moderation, like perhaps no more than the eight ounces per day.
Additionally, the drinking water standard is also the cooking water standard, and when foods are simmered for long periods, like pasta sauce, stews, many soups, and similar foods, the arsenic does not boil away, but rather concentrates in the food. Thus, a long cooked pasta sauce that has been reduced by two thirds would, at the legal maximum level allowed could contain 30 ppb of arsenic.
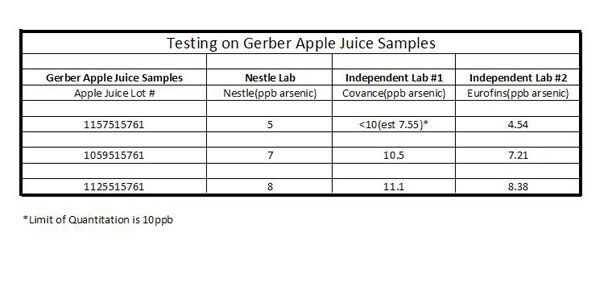
There is another bogus set of data presented on the Oz site. In it, laboratory results done by others is presented. Look at these results before you read the next paragraph and see if you can find the problem. It turns out that the table was not text but rather a picture, but I included it in the blockquote.
Nestle and two independent labs conducted a study on Gerber apple juice samples. Their results indicated the parts per billion (ppb) of arsenic found in these juices. For reference, the EPA has a limit of 10 parts per billion of arsenic in drinking water.
Did you see the issue? The results from Independant Lab #1 are qualified that “the limit of quantitation is 10 ppb”, This laboratory correctly presented the data, with an estimate of 7.55. The LOQ for the other laboratories are not presented, and the results are all lower than those reported by Lab #1. In addition, the Nestle laboratory and Lab #2 have values that are very close to each other, while Lab #1 has values that are 34%, 33%, and 28% higher than the Nestle one. This suggests to me that the results from Laboratory #1 likely suffer from a positive bias, in particular because the results from the other two laboratories are, to one significant figure, IDENTICAL. From these limited data I would come to the conclusion that none of the samples exceeded the drinking water standard, but I would need more data to say that with certainty.
There is an additional problem with Oz’s methodology. In his study, total arsenic was determined, and the distinction betwixt inorganic arsenic (the more readily absorbed, and thus more toxic kind) and organic arsenic was not made. To explain why is easy. Total arsenic is determined by oxidizing all of the arsenic in a sample to the inorganic state and then determined spectrophotometrically, usually by inductively coupled plasma spectrophotometry. This a fast and cheap way to determine many elements, but it says nothing about its native state.
To determine organic arsenic, either or both of the techniques of gas chromatography/mass spectrometry (GCMS) or liquid chromatography/mass spectrometry (LCMS) are required. These are much more demanding and much more expensive and time consuming than ICP.
The methodology argument is not original with me. Actually, FDA itself made the argument originally a few days ago, and many other, independent experts agree with FDA. Here is a link to a report about the FDA response. Two salient statements are as follows:
The FDA believes that it would be irresponsible and misleading for The Dr. Oz Show to suggest that apple juice contains unsafe amounts of arsenic based solely on tests for total arsenic.
, and
In a second letter to the show, Zink informed the producers that the FDA had performed its own testing on samples of apple juice from the same lot that yielded the highest level of arsenic in Dr. Oz’s investigation. All of the results ranged from 2 ppb to 6 ppb.
Unfortunately, the report cited does not indicate whether FDA was looking at total arsenic or just organic arsenic, and I would like to know more, but the bottom line is the same. Oz has gotten a lot of attention for what is essentially bunk science. My personal opinion, and this is not scientific but rather emotional, is that Oz has a lot of P. T. Barnum in him and will sell “hokum” for money, regardless of the cost. The few times that I have seen him on TeeVee, he has come across as very unauthentic. Once again, that is a personal opinion. By the way, this is not the only questionable activity in which Oz has participated, but this piece is about arsenic.
Well, you have done it again! You have wasted many einsteins of perfectly good photons reading this toxic piece! And even though Bachmann changes her mind and decides that protecting girls from contracting cervical cancer in later life is a good idea when she reads me say it, I always learn much more than I could ever possibly hope to teach by writing this series! Therefore, please keep those comments, questions, corrections, and other feedback coming! Remember, no science or technology comment is ever off topic here, regardless of the topic of the evening. Tips and recs are also appreciated. I shall hang around tonight as long as comments warrant, and shall return tomorrow after Keith’s show for Review Time. By the way, he still needs to contact me about that Science Adviser gig for Countdown.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
firefly-dreaming, and

2 comments
Author
two toxic subjects?
Warmest regards,
Doc
Author
I very much appreciate it!
Warmest regards,
Doc