(9 pm. – promoted by ek hornbeck)
Last time we talked about fluorine, the very most reactive chemical element. Now we add a single proton to the fluorine nucleus and come to Element 10, the LEAST reactive chemical element. What a difference a charge can make!
Actually, neon is quite common in the cosmos but quite rare on earth. It is fifth, after the elements that we have already discussed, because it is mostly a light even/even nucleus. But that is not what makes it outstanding.
There are three stable isotopes of neon, 20Ne, at almost 91% natural abundance on earth, 21Ne, at about a quarter on one per cent, and 22Ne, the remainder. This gets important later.
The reason the 20Ne is so common is that it is easy to make in large stars. Just the alpha process, viola, you fuse an one oxygen and one helium together and get neon. Both of those elements are quite common, so the process is also common.
21Ne is mostly produced from other elements here on earth from either nuclear decay of heavy elements or from cosmic ray bombardment of air. The around 10% of 22Ne is from neutron irradiation of 25Mg, which is also stable and at around 10% natural Terran abundance for magnesium isotopes.
So, in space there is a LOT of neon. On earth it is quite rare, much rarer than its cosmic abundance would betray. There is a very simple reason for that.
Neon is completely inert, meaning that it forms no stable compounds with any other element or even itself. Some quasi compounds can be made, but only under conditions that involve ionization, and neon is hard to ionize. Under normal conditions neon does not combine with anything, and after extreme conditions are removed, any species containing neon decays back to neon and what ever else was associated with it.
The reason for this is that the L electron shell is completely filled. There are 2 2s and 6 2p electrons, forming an octet, and an octet is extremely stable electronically. Neon is also relatively light, with a mass number of 20 to 22. Thus, when the inner planets were forming there was nothing to keep neon around in the form of refractory compounds, and the neon just boiled away into space.
Go back to the link before and look at the video where they construct the molecular orbital diagram for fluorine. Remember, each neon atom has one more electron than fluorine, so the final pair of electrons has to go into the sigma* antibonding orbital, giving a bond order of zero for Ne2, so there is no stability to be gained for this configuration and thus neon is monatomic.
Neon was discovered in 1898 along with krypton and xenon. Helium was already known, as was argon. Thus, except for radon, all of the noble gases were accounted for in 1898. The way that they were isolated was to take a huge amount of liquid air and distill it. As I recall they removed the oxygen by combustion to simplify their task. It took lots of liquid air to provide enough neon to be able to work with, because neon in the atmosphere amounts only to 18 parts per million v/v. Thus, to get one mL of the gas, you would have to distil almost 56 L (about 14.5 gallons) of air to get it. Still, except for argon, neon is the most common noble gas in the atmosphere.
Thus, we are all breathing neon in and out constantly, but since it is completely nonreactive in biological systems and is present in amounts way too small to present an asphyxiation hazard, it is completely harmless.
We are most familiar with neon in neon signs, the familiar orange red glow. Neon under reduced pressure is ionized to create a plasma that is electrically conductive, and energy passing through the plasma is transferred to other, nonionized neon atoms to produced excited states that, when they drop back down to the ground state, emit photons of particular colors. Here is a spectrum on neon from Wikipedia:
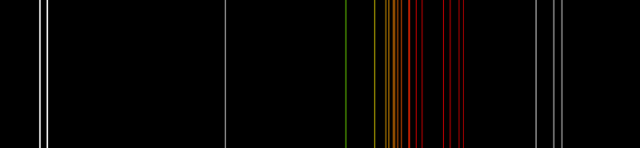
The white lines at the very left and right are the invisible ultraviolet and infrared emissions, respectively. The colored lines in the middle are the visible emissions of neon, and you can see that all but one are in the yellow, orange, and red. That is what gives neon lights their characteristic color.
Neon has a few uses other than for signs, but if neon signs or lights are what you’d like to see it used for then you can visit sites like NeonMama.com to find them. On the subject of lights, I have a circuit tester with a neon bulb that glows when a circuit is energized, and I suspect that many of you do as well. Since it is the most inert element, it is used in several applications where it is important to be completely nonreactive, such as high tension electrical switching equipment.
It can be used as a cryogenic refrigerant, since its boiling point is 27 K, or -246 degrees C. However, it is much, much more expensive than helium and is used only when the extremely low temperature of liquid helium (4.2 K) causes problems.
Neon played an extremely important role in explaining why elements do not usually have integral mass numbers, but rather a nonintegral one close to but heavier than where it should be. See, before 1913 isotopes of elements were unknown.
That year Francis Aston and J. J. Thompson, brilliant British physicists, were experimenting with canal rays, which are just positively charged ions. In a high voltage discharge tube, electrons are stripped from atoms and go one direction, whilst the positive ions go the opposite direction. Aston and Thompson wanted to see if these rays were deflected by magnetic and electric fields like electrons are, so the put a photographic plate (the only detector other than the human eye at the time) at the end of a discharge tube filled with neon and then put the tube betwixt the anode and the end where the plate was betwixt the plates of an electrical capacitor and betwixt the poles of a magnet.
Sure enough, once the discharge tube was energized the positive ions DID respond to those fields and their normally straight line of flight was curved depending on the strength of electric and magnetic fields. It was already known from other studies that neon has a mass number of a little over 20 atomic mass units (amu), and they did see a strong track at the corresponding position once the plate was developed. But what else they saw was extremely interesting.
At the position corresponding to 22 amu, a faint signal was also seen. Since it is possible to integrate the signal on a photographic plate by (at the time) scraping away and weighing the exposed, developed emulsion, relative abundances can be estimated with some degree of accuracy. Aston and Thompson determined that the amount of the signal at 22 amu was about 10% of that of the signal at 20. When the weighted average of those two signals is determined, it comes out to be remarkably close to 20.2 amu, the experimentally determined value by direct measurements on pure neon.
This was a profound discovery, and it revolutionized the understanding of the atom. Until then the conventional wisdom was that all atoms of a given element were identical in all respects. This observation turned that though on its head, and also the model of the atom. Up until then the atom was thought be be composed of negative electrons surrounding a positive nucleus composed of what are now called protons.
Ernest Rutherford had only demonstrated the existence of the nucleus in 1911, and just two years later the picture became much more complicated than previously thought. In 1920 Rutherford himself, using the data from Aston and Thompson, postulated that the nucleus might contain massive neutral particles in addition to protons, and that explained everything. It was not until 1932 that James Chadwick conclusively showed that there were indeed neutral particles in the nucleus of about the same mass as the proton, and Chadwick called them neutrons.
Back in undergraduate school I wrote a paper for my wonderful History of Chemistry class about this very topic but unfortunately the cuneiform tablet got broken. The gist of the paper was that there was a collaborative genius during this era that completely changed our understanding of physics betwixt around 1898 and around 1932. Thus, our understanding of the very fundamentals of how matter is assembled was revolutionized in around one human generation, a truly astounding figure.
Here are a few facts about neon. Lasers using helium and neon are common, and were once used for reading optical disks (the old Laserdisk format). Now laser diodes are used for these applications in most consumer products, but HeNe lasers are still extremely useful for technical and industrial work because they can be built to provide more power than laser diodes. The diodes contain no neon, but have an output wavelength very close to the HeNe lasers in consumer products because of compatibility reasons.
There is only one terrestrial source for neon, the air. At 18 ppm it is expensive to produce, compared to helium which can be found in the per cent ranges in some gas wells. Neon can be neither created nor destroyed (except by some nuclear processes which are not commercially viable), so what is used goes back to the atmosphere when released. Neon, being less dense than air, is slowly evaporating into space, but not nearly as quickly as helium does. Thus, neon is essentially renewable forever whilst helium is lost once it is released.
There IS helium in the atmosphere, but only at around 5 ppm, less than one third the concentration of neon. Since the gas wells deliver per cent levels, it is not economic to try to recover helium from the atmosphere, but that may have to be considered as supplies dwindle. It may be that membrane technology will be improved to the point that the noble gases can be separated with lower energy costs, but at such low concentrations at least some preconcentration would have to be done.
Overall, neon is not used nearly as extensively as helium, mostly because of cost. Where it is used, few if any substitutes are to be had. Enjoy the orange red lights and do not worry that they are depleting the supply of neon, because we get it all back when the glass breaks.
That about does it for tonight. If I write about elements next week, sodium will be at bat, but I may shift gears for a week or two and address other technical issues, depending on what strikes my interest.
Well, you have done it again! You have wasted many more einsteins of perfectly good photons reading this inert piece. And even though Mitt Romney realizes that he is fooling no one with his Friday new dump about his tax numbers when he reads me say it, I always learn much more than I could possibly hope to teach writing this series. Thus, please keep those comments, corrections, questions, and other feedback coming. Tips and recs are also always welcome, too. Remember, no science or technology issue is off topic here.
I shall most likely be late for Comment Time as it looks like someone and I are going to be able to spend some quality time together tonight, but I promise to check in when she conks out this evening. It was 11:45 Friday when she did, so I hope to be quite late. In any event I shall return tomorrow evening around 9:00 Eastern for Review time.
Warmest regards,
Doc, aka Dr. David W. Smith
Crossposted at
Daily Kos, and

2 comments
Author
the most inert element known?
Warmest regards,
Doc
Author
I very much appreciate it.
Warmest regards,
Doc